
Kerri Messinger’s angiogram brought frightening news.
She was lying on an exam table at Robert Wood Johnson Medical Center in New Brunswick looking at images of her brain. Endovascular neurosurgeon Gaurav Gupta explained the findings.
“He’s showing me on the screen, ‘This part is normal but this—this is a problem. This is an aneurysm,’” she recalls.
Gupta didn’t have to explain to Messinger that a brain aneurysm, a bulge in the weakened wall of a blood vessel, could burst and cause a stroke—or that it could take her life.
She knew. Her father had died suddenly from a brain aneurysm when Messinger was a child. She’d had two aneurysms herself, leading to open-brain surgery in 2008. That had involved an incision from her forehead to behind her ear, the temporary removal of part of her skull, and an only partly successful attempt to clip off the blood supply to the affected vessel. By 2013, the aneurysm had reached a dangerous size.
What went through her mind as Gupta spoke was her prolonged struggle to recover from that 10-hour operation. Messinger, then 38, had gone into kidney failure afterwards, developed blood clots, and had lingering problems with speech and vision. She’d spent weeks in the hospital and in rehab, missed months of work, and sought treatment for depression because she felt dependent and debilitated. She had needed extensive physical therapy simply to be able to walk unassisted.
But things had gotten so much better. Back at work at the Hunterdon County Library, she could once more drive the bookmobile. She had a wonderful boyfriend. Could she face that whole ordeal again?
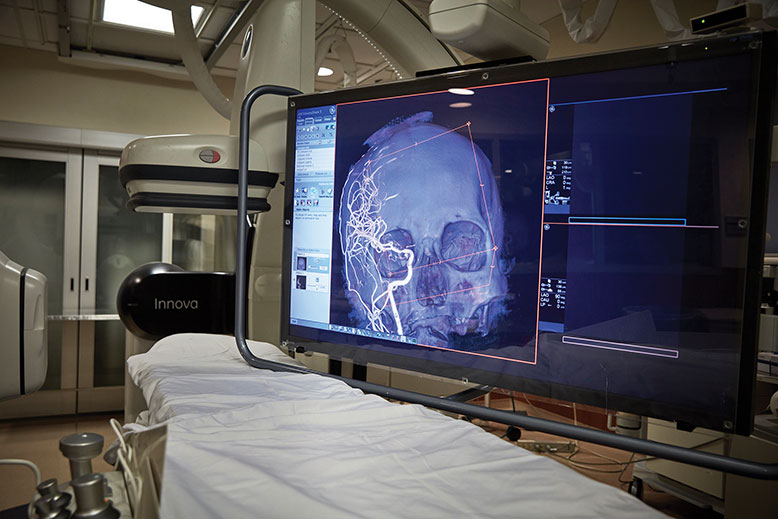
The Neurointerventional Suite at Robert Wood Johnson University Hospital where surgeons can view live, 3-D images of a patient’s brain on a high-definition monitor. Photo by Matt Furman
“I can’t do it,” she told Gupta. “I can’t go through brain surgery again, dealing with all the complications.”
He touched her arm reassuringly. “Kerri, you don’t have to go through that again,” he said. “There’s a new procedure.”
For decades, New Jersey patients looking for cutting-edge medical treatment frequently wound up in Philadelphia or New York.
One reason is that new devices, drugs and techniques usually get tested first at academic medical centers. The chance to undertake such investigations—experiments, pilot studies and then larger clinical trials—is a major reason physicians want to work at such institutions.
“That’s part of our core mission,” says Dr. Eric A. Singer, who directs the Distinction in Bioethics program at Rutgers Robert Wood Johnson Medical School. “We’re here to do research, to ask questions and come up with ways to answer them.”
While medical schools in Philadelphia and New York date to the 18th and 19th centuries, New Jersey’s are far newer. The first class at Rutgers Medical School entered in 1966. A series of consolidations created the College of Medicine and Dentistry in 1971, which in 1981 gained university status; most of it is now part of Rutgers. The Cooper Medical School of Rowan University graduated its first class in May.
That growth is helping the state get over its longtime medical inferiority complex.

Vascular surgeon Jeffrey Carpenter, chairman of surgery at Cooper Medical School, was the first practitioner in the country (with his colleague, Dr. Francis Caputo) to treat an aortic aneurysm with a device called the Nellix. Photo by Matt Furman
“A lot fewer people probably feel that way now,” says Dr. Jeffrey Carpenter, who left the University of Pennsylvania after 23 years to become Cooper Medical School’s first chairman of surgery. “Frankly, there are things we do here now that you can’t find in Philadelphia.”
So while Kerri Messinger had the bad luck of a recurring aneurysm, she was in the right place at the right time. By 2013, when she met Gupta, the Food and Drug Administration had approved a new stent for use in treating brain aneurysms. Gupta and his colleague, neuroradiologist and director of interventional neuroradiology at RWJ Sudipta “Sid” Roychowdhury, were the first in the state to use it.
“This is a blood vessel that goes into the brain,” said Gupta, drawing a narrow pathway on a pad. He added a bulge: the aneurysm.
“It’s like a ballooning in your tire,” Roychowdhury explained.
Not everyone who has a brain aneurysm needs surgery. But if one ruptures, causing a hemorrhagic stroke, “it’s a devastating event,” he went on. About 40 percent of victims die; survivors usually suffer irreversible brain damage. Where rupture appears likely, neurosurgeons try to seal off the bulge before the wall gives way.
Open-brain surgery like Messinger’s 2008 procedure has since largely yielded to minimally invasive techniques. Surgeons can insert a catheter through the groin and thread it up through the aorta and carotid artery into the brain, where they insert a small metal coil or a stent.
But those procedures don’t work as well for larger aneurysms in hard-to-reach places like Messinger’s, which had reached the diameter of a dime. Those patients’ aneurysms often recur, requiring repeat surgeries.
Now, Gupta and Roychowdhury use the Pipeline stent, FDA approved for such difficult cases.
Gupta brought a sample, still encased in its plastic catheter. As he pushed from its base—during an actual operation, he’d be watching on an X-ray monitor—the stent, braided from two metals, emerged from the far end of the tube. It resembled a tiny, silver Slinky.
“In the brain, the roads are not straight,” he explained. The mesh Pipeline bends and expands so “it can conform to the irregularities and curves.” Once it’s in place inside the blood vessel, “you’ve got a strong wall that diverts the flow of blood away from the aneurysm.”

The Pipeline stent was FDA approved in 2013 for treating brain aneurysms. The Slinky-like device bends and expands to conform to the irregularities and curves of a blood vessel. Photo courtesy of Metronic
Studies to date, though still small-scale, show that aneurysms are far less likely to recur with the Pipeline; the rate of complications appears acceptably low. Compared to the coil method, Roychowdhury said the Pipeline stent “has similar risk; however, the ability to get to a sustained cure the first time around is higher.”
Gupta and Roychowdhury have performed about 200 such surgeries (a handful of other New Jersey hospitals now use the Pipeline, too); one was Kerri Messinger’s.
This time, the procedure took less than half as long as her previous operation. She spent two nights in the hospital, stayed home from work for a week, and felt a bit of groin soreness from the catheter. To her relief, that was it.
Now 44 and living with her boyfriend on a Flemington farm, she still gets regular scans. But so far, “my aneurysm is totally healed,” she says. “It amazes me that in five short years, the technology has changed so much.”
Cancer treatment is also changing, but not yet so radically that specialists in the disease, like Itzhak Avital, a surgical oncologist, can cure patients with advanced cancer throughout the abdomens. What Avital can offer, with a technique that sends a heated brew of chemotherapy drugs sloshing directly into patients’ bellies, is the possibility—not the guarantee—of more time. “For some people, six months is everything,” he says.
Brian Snow, a 48-year-old sales director from East Brunswick, for instance, yearned to see his daughter graduate from high school. He underwent this treatment in May—known as cytoreductive surgery (CRS) with HIPEC (hyperthermic intraperitoneal chemotherapy)—after previous rounds of surgery and chemo failed to stop his colon cancer from spreading to his liver, gall bladder, small intestine and parts of his lungs.
“It’s never cured,” Snow says of his disease. “It’s always at bay.” But he watched his daughter get her diploma from East Brunswick High.
Avital began experimenting with heated chemotherapy 10 years ago at the National Cancer Institute. At the time, “this was a kind of rogue surgery, a procedure that pushed the limits, extreme,” he says. Since then, he’s performed it about 250 to 300 times at the institute, at a cancer center he led in Virginia, and then at St. Peter’s University Hospital in New Brunswick, where until recently he was chairman of surgery. He has resigned from St. Peter’s and plans to continue his work at another New Jersey Hospital, though he cannot yet disclose which one.
He has used the procedure (as have no more than two dozen surgeons elsewhere in the country) to treat colorectal cancer, cancer of the appendix, a disease called peritoneal mesothelioma and—more experimentally—advanced stomach, ovarian and uterine cancers.
It’s never the first-line treatment; patients who come to Avital have already had mainstream approaches fail. Even then, “I say no as often as I say yes,” Avital explains, because there’s too much cancer to remove or because the patient is too weak or has other life-threatening illnesses. He also requires patients to get second opinions because he understands their desperation.
“With these diseases, if you let nature take its course, you measure survival in months,” he says. His own pilot study of 17 patients with gastric cancer found that median survival with surgery and HIPEC was 11.3 months. With standard treatment, it was 4.3 months. Two of those pilot HIPEC patients lived for two years, one for more than four; the longest survival is over eight years now.
The surgery involves first painstakingly removing all visible tumors within the abdominal cavity, where cancer has typically spread to three or four organs. Then the surgeons temporarily close the incision and use catheters to dispense cancer-killing drugs into the abdomen, instead of dripping them into the bloodstream as standard chemotherapy does.
Heating this toxic soup to 43 degrees Celsius (about 109 Fahrenheit) appears to help the drugs penetrate deeper into tumors, Avital says; avoiding the bloodstream allows surgeons to use higher doses. Pumps help circulate the lukewarm liquid for 30 to 90 minutes, then drain it; the surgeons again open the patient, wash out the abdomen and restitch the incision. Patients typically begin standard chemotherapy about eight weeks later.
“You have to hate cancer—what it does to people, to loved ones, to society—to do a good job at this,” Avital says. At the outset, his HIPEC patients look profoundly sick: malnourished, nauseated, usually in pain, bellies distended with tumors. When they return after surgery, he says, “you see the transformation from hopeless to hopeful.”
Still, it’s important to view new approaches with caution. What looks effective in early studies doesn’t always pan out in larger clinical trials. An apparent breakthrough, with good results after a year’s follow-up, may have less impressive outcomes five years later.
“Is this new procedure an improvement over what we have been doing?” says Singer, director of the bioethics program at Rutgers. “With a new suture or a topical cream that reduces scarring, you can tell fairly quickly. If we’re looking to change the course of a disease, it might take years or decades.”
“We pursue new technologies, but with some caution,” Roychowdhury agrees. “I’ve seen so many things that haven’t worked.”
Patients interested in experimental procedures receive extensive counseling and stacks of consent forms. Some say no, thanks. “They want to know that something is tried and true,” says Carpenter, chair of surgery at Cooper. But for others, the chance to advance science, even with its inevitable stumbles, becomes a reason to enlist.

The Nellix, an investigational device used to treat aortic aneurysms. Cooper Medical School is one of 26 clinical trial sites for the Nellix, which is seen as less likely to cause leakage from the aorta than previous stents. Photo courtesy of Metronic
Take Michael Barte, a Forked River retiree given the opportunity to be the country’s first recipient of the Nellix, an investigational device used to treat aortic aneurysms. When Barte and his wife met with Cooper surgeons, “they gave me every pro and con you can think of,” he says. He decided, “Somebody’s got to be the guinea pig.”
An aneurysm in the aorta, the body’s central artery, can be even more lethal than similar bulges in the brain, Carpenter points out. If it ruptures, half of victims die before they get to a hospital, and only half of those who reach hospitals survive.
Carpenter holds a life-sized plastic aorta against his body to show where aneurysms occur. Like brain aneurysms, aortic aneurysms are now commonly treated with minimally invasive catheters and stents instead of open surgery. The problem: Stents too often shift within the aorta, causing leaks and requiring additional surgeries.
Enter the Nellix. Carpenter, a vascular surgeon and inventor (he holds several patents on surgical devices), and his partner and fellow investigator, Dr. Francis Caputo, were the first in the country to treat an aortic aneurysm with the Nellix, at Cooper in 2014.
Carpenter serves as global principal investigator for a multi-site clinical trial of the Nellix, which appears to offer a key advantage. After surgeons position the experimental stent, closing off the bulge in the aorta, they inject a liquid polymer that quickly solidifies around it. “It has the consistency of an eraser on a pencil,” he says. “It makes a tight seal.” The seal holds the stent in place, reducing leakage.
Thousands of patients have received Nellix systems in Europe, where regulatory approval for new devices is easier to get, but Barte became the first American when Carpenter and his colleagues operated in 2014. Cooper, one of 26 clinical trial sites in the United States, has since performed 25 more procedures.
So far, Barte is doing well; so is the Nellix. In its first year, Carpenter says, leaks and re-interventions are significantly lower than with standard stents. Endologix, the company that markets the device, expects FDA approval next year.
“Surgeons leave the operating room every day contemplating, ‘How could I have done that better?’” says Carpenter, himself an inveterate tinkerer. “‘If I only had this device…’” He and his wife, Judith, also a physician, have designed several of their own, working at their kitchen table.
Carpenter is proudest of one inspired by—of all things—a Fourth of July cocktail party at his parents’ home in Ocean City. At the time, more than a decade ago, he was thinking about a recently introduced operation to replace damaged heart valves. It was less invasive than open-heart surgery, but it could also dislodge debris (called emboli) from arteries, which too often flowed into the brain and caused strokes. As a surgeon, “You lay awake at night thinking, How can I keep that from happening?”
His mother was passing around drinks with little paper parasols that opened and closed. What if a similarly shaped deflector could be introduced through a catheter, trap emboli during valve-replacement surgery and protect the patient’s brain, then be withdrawn afterwards?
The Carpenters patented the resulting Embrella (for embolic umbrella) in 2006, raised money and formed a company to perfect the device. He observed its first human use at a Vancouver hospital in 2009. After selling the company for a hefty sum in 2011, the Carpenters paid off the mortgage on their own Ocean City retreat, the House that Embrella Built—but the FDA has yet to approve the device.
They’re working on their next invention, nonetheless. No, they won’t say what it is.
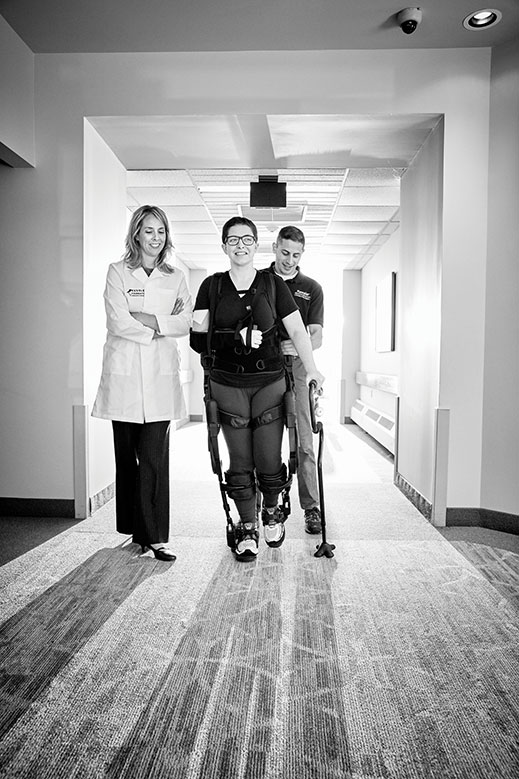
Assisting with her therapy are senior research scientist Karen Nolan and therapist Adam Kesten. The Ekso allows Yurek to practice the complicated sequence of neuromuscular actions involved in walking. Photo by Matt Furman
Laura Yurek is walking slowly down the hall at the Kessler Institute in West Orange. The robotic exoskeleton supporting her makes her look like a Transformer, but so what? “It feels great,” she says.
Yurek, 38, a Morris Plains nurse and homemaker, developed bleeding in her brain last winter when a tangle of blood vessels called an arteriovenous malformation (AVM), burst. She has spent weeks at Kessler, working to regain the use of her legs and right arm.
On her own, Yurek can’t stand. A traditional physical therapy session requires three therapists, two on rolling stools guiding the movements of her legs, one helping her balance. With such intensive assistance, she has managed to walk 80 feet in 25 minutes.
But with this contraption called the Ekso Bionics GT, and a single therapist behind her cueing her movements and monitoring the Ekso’s computer readings, she has walked for 52 minutes and logged 1,503 steps—cause for celebration.
The plastic-and-metal device holds her upright in proper alignment. Velcro straps connect it to her thigh and legs; foot plates slide beneath her sneakers. With motors at the hips and knees, powered by lithium-ion batteries in a backpack, the Ekso allows Yurek to practice the surprisingly complicated sequence of neuromuscular actions involved in everyday walking.
“Ready with your right foot?” says Adam Kesten, the therapist behind her. “Okay, one, two, three—step.” She’s working hard (he is, too), but when Kesten asks how she feels, Yurek says she wants to keep going.
Karen Nolan, senior research scientist at the Kessler Foundation, always aims to get patients moving sooner and further. “The more steps patients take, the more benefit they get,” she says. Originally, the Kessler team used the Ekso for patients with spinal cord injuries, but Nolan has just received a $1 million federal grant to explore its use with stroke survivors, who are far more numerous. She began enrolling the first of 96 patients in the study this fall.

Laura Yurek lost the use of her legs and right arm as a result of a brain bleed last winter. She is trying to walk again at Kessler Institute in West Orange with the help of a robotic exoskeleton called Ekso Bionics GT. Photo by Matt Furman
After a stroke, as with Yurek’s AVM, it’s the brain that has sustained damage, not the limbs. “The brain’s ability to heal is remarkable,” says Nolan. “We want to see if we can apply technology to help the brain heal faster, maybe even better, and recover more function.”
At an average $125,000, the exoskeleton will be used for research with inpatients and outpatients; nobody gets to take one home as a personal mobility aid.
Nolan’s early research shows that patients become more engaged in their therapy with the machine, however. They’re not just taking a robot ride; they’re rebuilding their neurological abilities. Still, she cautions, despite the exoskeleton’s early promise, “we don’t yet know how it’s going to affect long-term recovery.”
Researchers have to maintain skepticism and a careful neutrality, but patients are free to express their enthusiasm. Yurek’s two eldest children had come to visit her at Kessler a few days earlier, and there was their mom—moving deliberately and resembling Robocop, but walking.
“I was thrilled,” she says. “They were thrilled.”
Paula Span writes the New Old Age column for the New York Times and teaches at the Columbia University Graduate School of Journalism. She lives in Montclair.




Aneurysms are complex and enigmatic challenge to surgeons, clipping is one of the most crucial method for resolution. For more details: http://surgicalunits.com/aneurysm-clip-311.html